|
|
|
|
|
| Contact |
|
 |
 |
 |
|
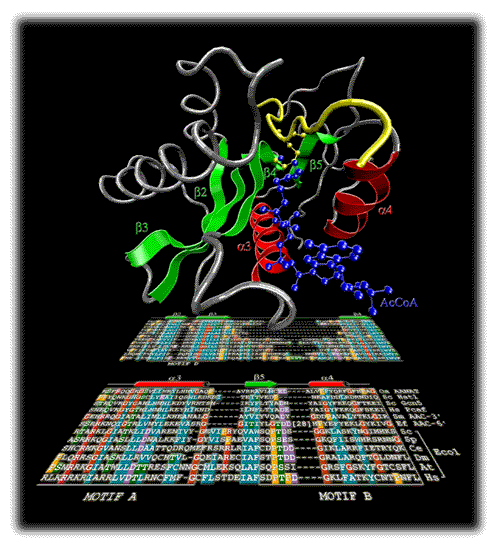
The cover image is an artist's view of how our understanding of protein structure and function is emerging from a sequence alignment. It shows the joint multiple alignment of Eco1 sequences from different organisms and sequences of members of the GNAT superfamily of acetyltransferases. The alignment was obtained based on domain fragment searches, secondary structure predictions and physico-chemical similarity of amino acid types. The three-dimensional structure of GCN5 histone N-acetyltransferase from Tetrahymena thermophilum [Rojas et al. Nature 1999; 401:93-98] is shown above. The secondary structure elements of the most conserved motifs D, A, and B that were predicted in Eco1 are colored. Eco1 is a protein that has been previously implicated in the establishment of bridges between sister chromatids during DNA replication. The unexpected acetyltransferase activity of Eco1 is reported in the paper by D. Ivanov et al. : | |||
|
Ivanov D, Schleiffer A, Eisenhaber F, Mechtler K, Haering CH, Nasmyth K. Eco1 is a novel acetyltransferase that can acetylate proteins involved in cohesion. Curr Biol. 2002 Feb 19;12(4):323-328. PMID: 11864574
The authors gratefully acknowledge support in creating the cover illustration by Sebastian Maurer-Stroh and Hannes Tkadletz. The program VMD was applied for molecular visualization (William Humphrey, Andrew Dalke, and Klaus Schulten: VMD - Visual Molecular Dynamics. Journal of Molecular Graphics 1996; 14:33-38). |
||||