- As an oncofetal mechanism that is mostly downregulated in the healthy adult, targeting of angiogenesis should lead to minimal side effects even after prolonged treatment.
- tumor-associated angiogenesis is a physiological host mechanism and its pharmacological inhibition should, consequently, not lead to the development of resistance
- each tumor capillary potentially supplies hundreds of tumor cells and the targeting of the tumor vasculature should, thus, lead to a potentiation of the antitumorigenic effect
- in contrast to the interstitial location of tumor cells, direct contact of the vasculature to the circulation allows efficient access of therapeutic agents.
 |
 |
 |
| Home | Initium | Angiogenesis | FAQ |
| Molecular Characterisation of Angiogenesis |
|---|
Molecular Mechanisms Considerable insight in the molecular and cellular biology of angiogenesis has been obtained by in vitro studies using endothelial cells, isolated from capillaries or large vessels. Angiogenic factors (heparin binding peptide growth factors: VEGF, FGF-1, FGF-2, PDGF, HGF/SF; non-heparin binding peptide growth factors: EGF, TGF-alpha, TGF-beta; oligosaccharides; inflammatory mediators: TNF-alpha, ILs, Prostaglandins; hormones: Oestrogens, Proliferin; cell adhesion molecules: VCAM-1, E-selectin (Klagsbrun et al, 1999)) are able to mimic in vitro all the steps of the angiogenic cascade, including endothelial cell proliferation, migration and differentiation (Daniel et al). | |
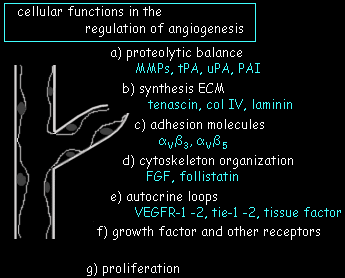 |
Angiogenic endothelial cells show a distinct genetic expression repertoir leading to modification of the principal cellular functions involved in angiogenesis. These are thought to fall into the following categories: a) regulation of the proteolytic balance leading to localized pericellular matrix degradation during cell migration, and protection against excessive extracellular matrix destruction. b) extracellular matrix (ECM) synthesis with preferentially expression of basement membrane components such as tenascin, type IV collagen, laminin, nidogen/entactin, and proteoglycans. c) synthesis of adhesion molecules involved in extracellular matrix interaction. d) cytoskeletal reorganization involved in cell migration. e,f) growth factors and their receptors. Indeed, our sequence analysis of a published angiogenesis expression profile experiment (St Croix B et al. Science. 2000 Aug 18;289(5482):1197-202) supports such a view. |
Antiangiogenic therapies
Antiangiogenic therapies have the following advantages that make the study of tumor angiogensis of practical interest:
Functional Genomics Approaches in Angiogenesis
PubMed: 10947988
Genes expressed in human tumor endothelium
St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW.
Science. 2000 Aug 18;289(5482):1197-202.
PubMed: 10854212
Gene expression profiling in an in vitro model of angiogenesis.
Kahn J, Mehraban F, Ingle G, Xin X, Bryant JE, Vehar G, Schoenfeld J, Grimaldi CJ, Peale F, Draksharapu A,Lewin DA, Gerritsen ME.
Am J Pathol. 2000 Jun;156(6):1887-900.
PubMed: 10792972
Identification of tumor angiogenesis-related genes by subtractive hybridization.
Wang JL, Liu YH, Lee MC, Nguyen TM, Lee C, Kim A, Nguyen M
Microvasc Res 2000 May;59(3):394-7
PubMed: 10769617
Gene targeting and gene transfer to unravel the molecular basis of the formation and disorders of blood vessels.
Carmeliet P.
Verh K Acad Geneeskd Belg. 2000;62(1):31-68. Review.
Last modified: Jan 2001
