|
|
|
|
|
| Contact |
|
 |
 |
 |
There are two distinct Smc2/Smc4-like complexes in worm (see also Tab. 2). One, consisting of the SMC proteins MIX-1 (an Smc2 orthologue) and DPY-27 (an Smc4-like paralogue), functions together with DPY-26 (see also discussion) in dosage compensation (Chuang et al., 1996; Lieb et al., 1996). The second complex involves MIX-1 and the true Smc4 orthologue and is required in mitosis (Hagstrom et al., 2002; Kaitna et al., 2002). The kleisin family member in this mitotic complex is so far unknown. Our analysis raised the possibility that the newly identified kleisin-β member might function in the mitotic SMC complex. To test this, we depleted the C. elegans orthologue KLE-2 (C29E4.2) by RNAi in a strain whose chromosomes are easily visualized due to expression of a histone GFP fusion protein. Embryos lacking KLE-2 exhibit severe chromosome segregation defects (Fig. 3). In wild type embryos, chromosome individualization is easily observed prior to nuclear envelope breakdown (NEBD). In kle-2(RNAi) embryos, in contrast, the chromatin appears uniform until NEBD. Finally, during anaphase, extensive chromosome bridging is observed and chromosomes fail to segregate properly to opposite poles. These phenotypes are indistinguishable from those seen in mix-1(RNAi) or smc-4(RNAi) embryos (Kaitna et al., 2002); thus, kleisin-β is involved in chromosome segregation. Its loss-of-function phenotype, together with the conservation of the N- and C- terminal kleisin domains, suggests that it might act in concert with MIX-1/SMC-4. Future studies will clearly be needed to verify whether it does indeed physically interact with these two proteins.
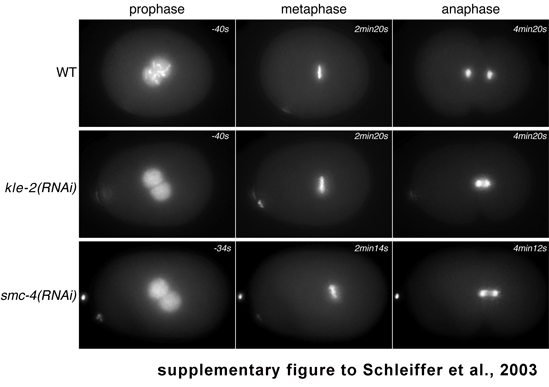
Figure Legend: KLE-2 RNA depletion in C. elegans
results in a severe chromosome segregation phenotype.
Embryos expressing GFP tagged Histone H2B were followed
through the first cell cycle by video microscopy. Indicated time-points are
relative to Nuclear Envelope Breakdown (NEBD). Deficiency of KLE-2
(C29E4.2 coding for the worm kleisin-β orthologue) RNA induced by RNAi
results in mitotic defects indistinguishable from defects observed in embryos
depleted of SMC-4. In prophase, chromatin fails to condense into individual
chromosomes. A well-structured metaphase plate is formed, albeit slightly
thickened compared to the wild type. In anaphase, chromosome segregation is
impaired, leading to the formation of extensive chromatin bridging.
Chuang, P. T., Lieb, J. D., and Meyer, B. J. (1996). Sex-specific assembly of a dosage compensation complex on the nematode X chromosome, Science274, 1736-9.[PUBMED]
Hagstrom, K. A., Holmes, V. F., Cozzarelli, N. R., and Meyer, B. J. (2002). C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis, Genes Dev 16, 729-42.[PUBMED]
Kaitna, S., Pasierbek, P., Jantsch, M., Loidl, J., and Glotzer, M. (2002). The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous Chromosomes during meiosis, Curr Biol 12, 798-812.[PUBMED]
Lieb, J. D., Capowski, E. E., Meneely, P., and Meyer, B. J. (1996). DPY-26, a link between dosage compensation and meiotic chromosome segregation in the nematode, Science 274, 1732-6.[PUBMED]